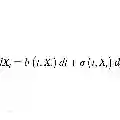Identifying objective neuroimaging biomarkers to forecast Alzheimer's disease (AD) progression is crucial for timely intervention. However, this task remains challenging due to the complex dysfunctions in the spatio-temporal characteristics of underlying brain networks, which are often overlooked by existing methods. To address these limitations, we develop an interpretable spatio-temporal graph neural network framework to predict future AD progression, leveraging dual Stochastic Differential Equations (SDEs) to model the irregularly-sampled longitudinal functional magnetic resonance imaging (fMRI) data. We validate our approach on two independent cohorts, including the Open Access Series of Imaging Studies (OASIS-3) and the Alzheimer's Disease Neuroimaging Initiative (ADNI). Our framework effectively learns sparse regional and connective importance probabilities, enabling the identification of key brain circuit abnormalities associated with disease progression. Notably, we detect the parahippocampal cortex, prefrontal cortex, and parietal lobule as salient regions, with significant disruptions in the ventral attention, dorsal attention, and default mode networks. These abnormalities correlate strongly with longitudinal AD-related clinical symptoms. Moreover, our interpretability strategy reveals both established and novel neural systems-level and sex-specific biomarkers, offering new insights into the neurobiological mechanisms underlying AD progression. Our findings highlight the potential of spatio-temporal graph-based learning for early, individualized prediction of AD progression, even in the context of irregularly-sampled longitudinal imaging data.
翻译:识别客观的神经影像学生物标志物以预测阿尔茨海默病(AD)进展对于及时干预至关重要。然而,由于潜在脑网络时空特性的复杂功能障碍,这一任务仍然具有挑战性,而现有方法往往忽视了这些特性。为解决这些局限性,我们开发了一个可解释的时空图神经网络框架来预测未来的AD进展,该框架利用双重随机微分方程(SDEs)对不规则采样的纵向功能磁共振成像(fMRI)数据进行建模。我们在两个独立队列上验证了我们的方法,包括开放获取系列影像研究(OASIS-3)和阿尔茨海默病神经影像学倡议(ADNI)。我们的框架有效地学习了稀疏的区域和连接重要性概率,从而能够识别与疾病进展相关的关键脑回路异常。值得注意的是,我们检测到海马旁皮层、前额叶皮层和顶叶小叶是显著区域,其腹侧注意网络、背侧注意网络和默认模式网络存在显著破坏。这些异常与纵向AD相关临床症状密切相关。此外,我们的可解释性策略揭示了既有的和新的神经系统层面以及性别特异性生物标志物,为理解AD进展的神经生物学机制提供了新的见解。我们的研究结果凸显了基于时空图的学习方法在早期、个体化预测AD进展方面的潜力,即使是在不规则采样的纵向影像数据背景下也是如此。